Readers are probably familiar with the idea of electrophoresis, although they may not know the term. The technique is used for DNA fingerprinting to determine paternity.
In television documentaries we often see forensic scientists holding a small X-ray film with lines of bar-codes. These bars are the physical locations of the genetic material after the DNA strands have been chemically separated, broken up and dragged through a viscous gel towards an anode. The bars mark the cumulative lodging place of many identical DNA pieces from many different cells.
We have the same DNA in every cell of our bodies, and DNA molecules are negatively charged. Each piece has a different physical resistance, so these bars mark the cumulative lodging place of many identical DNA genetic parts.
During the years of childhood and growth cells are constantly dividing and duplicating by a process called ‘mitosis’, so it is especially important that the DNA replicates accurately and that the gene sequences remain in order; these two-metre helical strands of paired molecules contain the basic blueprint for constructing and maintaining viable life.
There are 50,000 billion cells in the body, and even in older people the body is still actively creating another billion new cells every hour, so the incorruptibility of DNA is all-important in our health and survival.
Despite this constant manufacture of new cells, we don’t keep growing in size after adulthood. A few die from normal wear and tear (‘necrosis’) but, to maintain the balance, mis-copied or unwanted cells are instructed to suicide (‘apoptosis’) by the cells nearby.
Programmed cell death is an essential part of life, and, if this euthanasic message fails to trigger suicide and the cell goes into a phase of uncontrolled division, tumors and cancers result.
The cells of the heart muscle, and those of the nerves and brain neurones don’t replicate, but all others are reproducing regularly over your lifetime. So at the molecular-cell level there’s a new you about every five years.
This raises the question: Why do we get cancer? Cancer is slow in onset; it generally takes between ten and twenty years to incubate. Why do we get it at all if most cells are only five years old?
Obviously the defects which cause uncontrolled cell growth are often (but not always) transmitted from mother-cells to daughter-cells during mitosis. Defects like these are called ‘mutations’ Ñ however, not all mutations are disruptive or dangerous to our health. DNA in our cells constantly comes under attack from many sources, and the normal body processes ignore or handle most of the defects.
External messages are also transmitted across cell boundaries and between cells to initiate apoptosis (programmed cell death), but these may similarly be short-circuited or distorted in some way. These messages are carried by electrically-oriented flows of ions and by more complex protein and enzyme molecules.
The point is, that at the molecular level, humans cell functions are very dynamic, very regenerative, constantly being disrupted and repaired, highly tolerant of defects, and very much affected by electrical influences.
Recently the biomedical researchers have begun using a technique similar to DNA fingerprinting to investigate damage to DNA. This is called single-cell gel (SCG) electrophoresis or ‘comet assay’, and it is capable of finding defects in single cell exposed to toxic chemicals or ionizing radiation.
Our interest here is in whether this technique can detect damage to cell functions or DNA viability from low level radio waves. Classical radio theory says radio waves can’t damage molecules, because their energy is not sufficient to break chemical bonds.
The technique gets its name from the comet-and-tail appearance which results from broken genetic material being dragged through the gel by electrical attraction ahead of the more-resistant DNA bundles.
Think of this as towing a very old car through a few miles of deep mud, then counting the bits and pieces that fall off in the process. But here the car (the DNA ball) drags behind and the broken bits move out ahead.
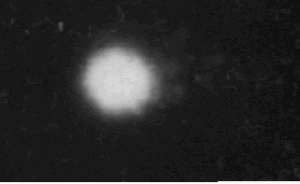
Fig.1 Unexposed control. The bundle is simply DNA.
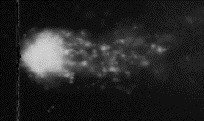
Fig.3 X-ray calibration: After 25.6 rads.
DNA strand breaks are now very obvious.
Comet assay techniques were developed by Swedish scientists Östling and Johannson in 1984, and then later refined by Narendra Pal (‘NP’) Singh in 1988 (with other improvements later). At that time Dr Singh was a research scientist at the US National Institute on Aging.
Chemical processes are employed to digest and remove all the lipids and proteins from the cell to express the DNA breaks, and Singh’s alkaline separation techniques are now widely recognized for their sensitivity and reliability. Alternative ‘neutral’ approaches are applied also in some research laboratories.
Comet assays reveal damage to DNA from air and water pollution, food additives, diet and smoking, etc. and they always require very highly developed laboratory skills and strict attention to detail. Unfortunately they lack a recognized form of objective measurement.
Back in 1994, Singh joined biomedical scientist Dr Henry Lai at the Bioelectromagnetics Research Laboratory, University of Washington in Seattle. The work originally conducted at this university was funded by the US Navy and Air Force, but that source of funding has long evaporated. Under Henry Lai, the US government’s National Institutes of Health has been responsible for most of the funding.
In a ground-breaking series of experiments between 1994 and 1998 they demonstrated convincingly that moderate levels of microwave (2.45GHz) radiation, ( Below that of cell phone radiation levels)for exposures of only two hours, could increase the frequency of single-strand DNA breaks in the brain cells of live rats.
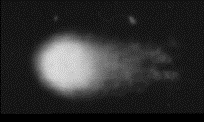
Fig.4 Assay showing effect of 2 hrs of microwave exposure (2.45GHz) at a SAR (absorption) level of 0.6 W/kg [about cell phone radiation levels] DNA strand breaks are also obvious.
Figure 4 was captured by Dr Lai and Singh, and it shows the results of a comet assay at power densities about one-fifth those previously thought to cause adverse biological effects. These exposures were only for a short time, and they used radio power-densities well below those said to be ‘ionizing’ (having the power to break chemical/material bonds).
In this research, Dr Lai and Singh have used microwave frequencies which are higher than cellphones (at 0.9GHz), but not much above those used by the cellphone cousins, the new handheld PCS phones (1.9GHz).
DNA strands tend to break all the time, but they repair themselves constantly, so these comet-tail images need to be compared with the unexposed control DNA bundle in Figure 1. The cell bundles in Figure 4 have the classic comet tail of particles indicating extensive DNA damage, well above the spontaneous DNA damage levels of the controls.
Spontaneous breaks in the DNA are relatively common in all cells (6.00-radical attacks seem to be responsible) and most are quickly repaired by normal cell processes — generally within minutes or hours. But any form of increased disruption to the DNA is worrying. Nerve cells in particular have a low capability for DNA repair and so the effects of additional breaks could accumulate.
The DNA strands form a spiral-staircase-like helix, and so breaks on only one side of the ladder are much easier to repair than those where both sides are broken. But in later experiments Lai and Singh found double-strand DNA breaks after similar exposures times and levels.
It is possible for the cell to make mistakes when repairing single-strand breaks, but the likelihood of serious mistakes (mutations) increases substantially with double-strand breaks.
Fortunately, only certain genes are ‘expressed’ (activated) within each organ, so less than one percent of the DNA is essential in any one cell. Most mutations will cause no harm, and those that are very disruptive will probably lead to programmed cell death.
This introduces a paradox; small problems accumulating over time may be more dangerous than large defects. Cells that suffer gross disturbances to their critical genes are also more likely be programmed to suicide; therefore the larger DNA disruptions may be self-annihilating.
Over the years the DNA in human cells constantly suffer attack, some of which is never repaired. Given enough time, the accumulation of minor (but jointly critical) problems can cause cancer to develop. There is rarely a single cause of cancer.
This is also why cancer is a mostly a condition of age. It’s probably that older people have many per-cancerous cells, even though only a few suffer the critical mutations that lead to uncontrolled cell proliferation. These are just the straws that finally broke the camel’s back.
This raises the distinct possibility that cumulative low level RF exposures could be more harmful than higher critical exposures.
And since nerve cells don’t divide and proliferate, this damage could equally contribute to degenerative diseases such as Parkinson’s and Alzheimer’s. Cancers and age-associated degenerative conditions may be closely related.
Another aspect of the Lai-Singh research (with pulsed microwave similar to GSM cellphones and radar) was also disturbing. Rat brains which were excised and prepared quickly for the assay showed fewer breaks, while those which were checked four hours after exposure revealed much higher levels. This suggests that both the damage and the repair-initiation are not simple and immediate processes, and supports the thesis that DNA damage from repeated uses of a cellphone could be cumulative.
Dr Jerry Phillips, working in a research facility outside Los Angeles, made a similar finding. His research showed that DNA breaks actually decreased in some RF exposure conditions, sometimes with different wave-forms, suggesting that there’s a more complex causal link than expected, and a delicate balance between the break and repair-rates.
Phillips work also suggests that there may be some type of rough feedback control mechanism — something like a sticky fly-wheel governor on a steam boiler which makes the engine-rate hunt between slow and fast. The DNA-repair feedback might lead to mistakes and mutation and increase the chance of destructive cancer.
This work is highly controversial, as you’d imagine. Lai and Singh have reported finding of DNA strand breaks at levels of only one-fifth the American RF safety limits — but they’ve since also found that they can use the pineal hormone melatonin and other anti-oxidants to countering the RF effect. So the research is not only producing negative results.
This points to the importance of free-radicals as the intermediary which actually damages the DNA, which doesn’t come as a surprise to most researchers. free-radicals have often been implicated in DNA problems.
Although the Lai-Singh research hasn’t been faithfully replicated, other scientists have found similar DNA strand breaks in parallel radio research projects, and a number of live-animal tests have confirmed increased tumor rates resulting from long exposures over the life of the animals. There is also evidence that radio-wave exposures can influence the short term memory.
Currently, the Lai-Singh research has been stymied for lack of funding from the US government which has its attention focused on other matters, while the cellular phone industry has preferred to invest in less disturbing projects.
